YAP and endothelin-1 signaling: an emerging alliance in cancer
$ 34.99 · 4.6 (68) · In stock
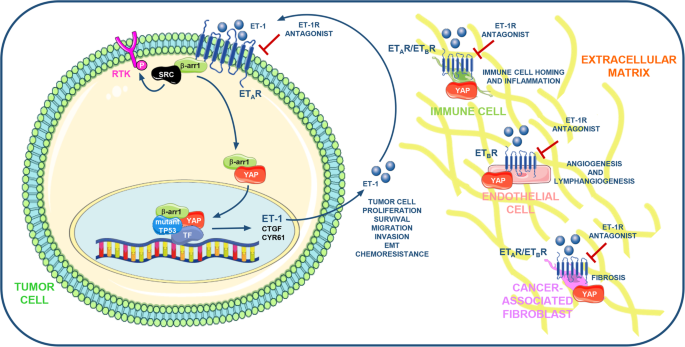
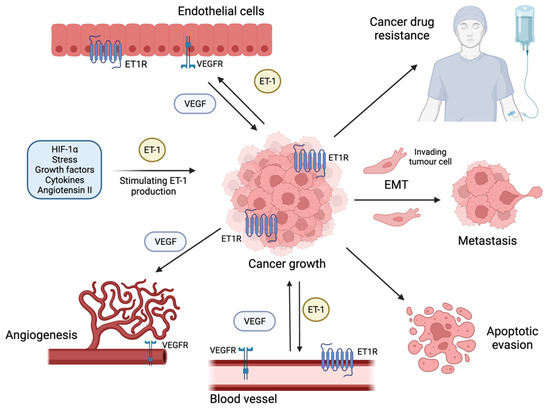
The rational making the G protein-coupled receptors (GPCR) the centerpiece of targeted therapies is fueled by the awareness that GPCR-initiated signaling acts as pivotal driver of the early stages of progression in a broad landscape of human malignancies. The endothelin-1 (ET-1) receptors (ET-1R), known as ETA receptor (ETAR) and ETB receptor (ETBR) that belong to the GPCR superfamily, affect both cancer initiation and progression in a variety of cancer types. By the cross-talking with multiple signaling pathways mainly through the scaffold protein β-arrestin1 (β-arr1), ET-1R axis cooperates with an array of molecular determinants, including transcription factors and co-factors, strongly affecting tumor cell fate and behavior. In this scenario, recent findings shed light on the interplay between ET-1 and the Hippo pathway. In ETAR highly expressing tumors ET-1 axis induces the de-phosphorylation and nuclear accumulation of the Hippo pathway downstream effectors, the paralogous transcriptional cofactors Yes-associated protein (YAP) and Transcriptional coactivator with PDZ-binding motif (TAZ). Recent evidence have discovered that ET-1R/β-arr1 axis instigates a transcriptional interplay involving YAP and mutant p53 proteins, which share a common gene signature and cooperate in a oncogenic signaling network. Mechanistically, YAP and mutp53 are enrolled in nuclear complexes that turn on a highly selective YAP/mutp53-dependent transcriptional response. Notably, ET-1R blockade by the FDA approved dual ET-1 receptor antagonist macitentan interferes with ET-1R/YAP/mutp53 signaling interplay, through the simultaneous suppression of YAP and mutp53 functions, hampering metastasis and therapy resistance. Based on these evidences, we aim to review the recent findings linking the GPCR signaling, as for ET-1R, to YAP/TAZ signaling, underlining the clinical relevance of the blockade of such signaling network in the tumor and microenvironmental contexts. In particular, we debate the clinical implications regarding the use of dual ET-1R antagonists to blunt gain of function activity of mutant p53 proteins and thereby considering them as a potential therapeutic option for mutant p53 cancers. The identification of ET-1R/β-arr1-intertwined and bi-directional signaling pathways as targetable vulnerabilities, may open new therapeutic approaches able to disable the ET-1R-orchestrated YAP/mutp53 signaling network in both tumor and stromal cells and concurrently sensitizes to high-efficacy combined therapeutics.
Biomedicines, Free Full-Text

The loss of endothelin-2 exhibits an anticancer effect in A549 human lung adenocarcinoma cell line

Box and Whiskers graph of ET-1 and ET-A expression versus lymph node

YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. - Abstract - Europe PMC
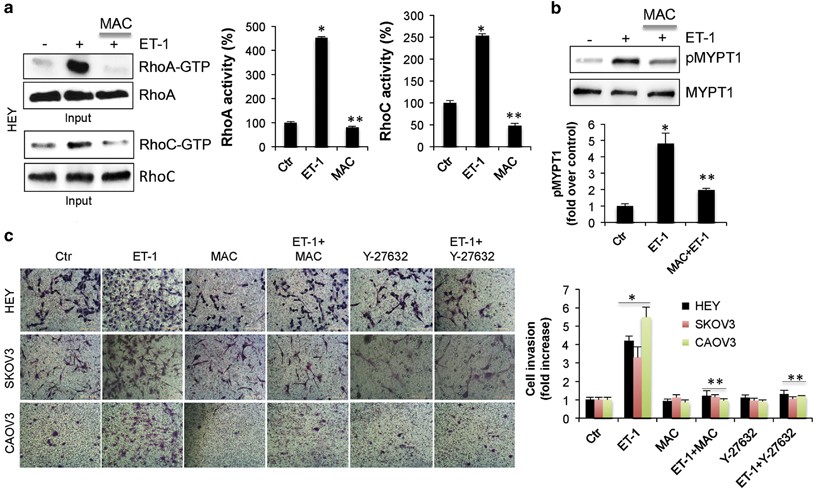
Endothelin A receptor drives invadopodia function and cell motility through the β-arrestin/PDZ-RhoGEF pathway in ovarian carcinoma

Irreversible and sustained upregulation of endothelin axis during oncogene-associated pancreatic inflammation and cancer
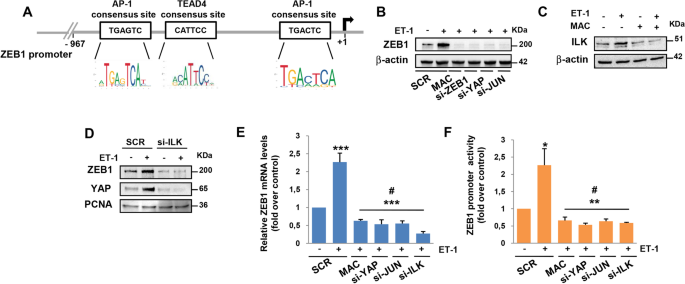
Functional interaction between endothelin-1 and ZEB1/YAP signaling regulates cellular plasticity and metastasis in high-grade serous ovarian cancer, Journal of Experimental & Clinical Cancer Research
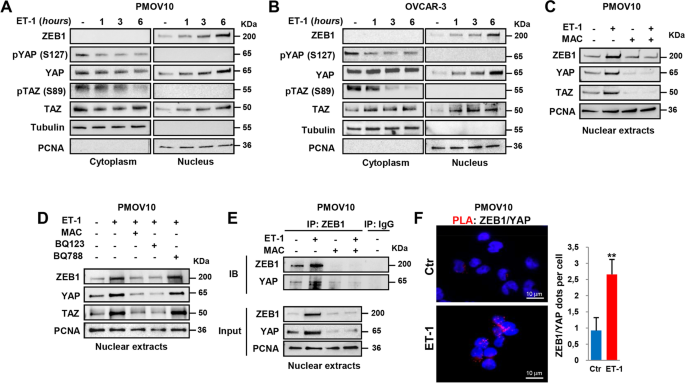
Functional interaction between endothelin-1 and ZEB1/YAP signaling regulates cellular plasticity and metastasis in high-grade serous ovarian cancer, Journal of Experimental & Clinical Cancer Research

Irreversible and sustained upregulation of endothelin axis during oncogene-associated pancreatic inflammation and cancer

Endothelin-1 axis fosters YAP-induced chemotherapy escape in ovarian cancer - ScienceDirect

Functional interaction between endothelin-1 and ZEB1/YAP signaling regulates cellular plasticity and metastasis in high-grade serous ovarian cancer, Journal of Experimental & Clinical Cancer Research

Targeting Endothelin-1 Receptor/β-Arrestin-1 Axis in Ovarian Cancer: From Basic Research to a Therapeutic Approach