Compression of a gas due to external pressure and the
$ 22.99 · 4.5 (458) · In stock


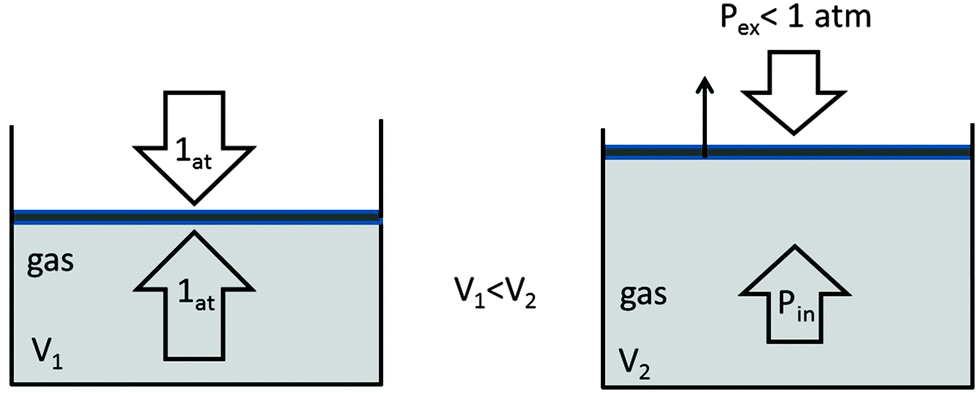
What will be the work done on an ideal gas enclosed in a cylinder, when it is compressed by a constant external pressure, pext in a single step as shown in Figure

Analysis of three scientific laws using the tool of Fig. 1

The work done in adiabatic compression of `2` mole of an ideal monoatomic gas by constant

1 (3) 248.5 K Pere R2- (4) 200 K La The work done in adiabatic compression of 2 mole of an ideal monoatomic gas by constant extemal pressure of 2 atm

How to Calculate the Work Done on a Gas Algebraically, Physics
Natural laws and ontological reflections: the textual and didactic

Cristian MERINO RUBILAR, Professor (Assistant)

thermodynamics - Immediate pressure change after compressing a gas - Physics Stack Exchange

An ideal gas undergoes isothermal compression from 5 m3 against a constant external pressure of 4 Nm-2. Heat released in this process is used to increase the temperature of 1 mole of
The fugacity of a gas is greater than its pressure. What can be said about the gas internal pressure? - Quora

6) The work done (kJ) in the irreversible isothermal compression of 2.0 moles of an ideal gas from 1 bar to 100 bar 25°C constant external pressure of 500 bar is (A)